Introduction
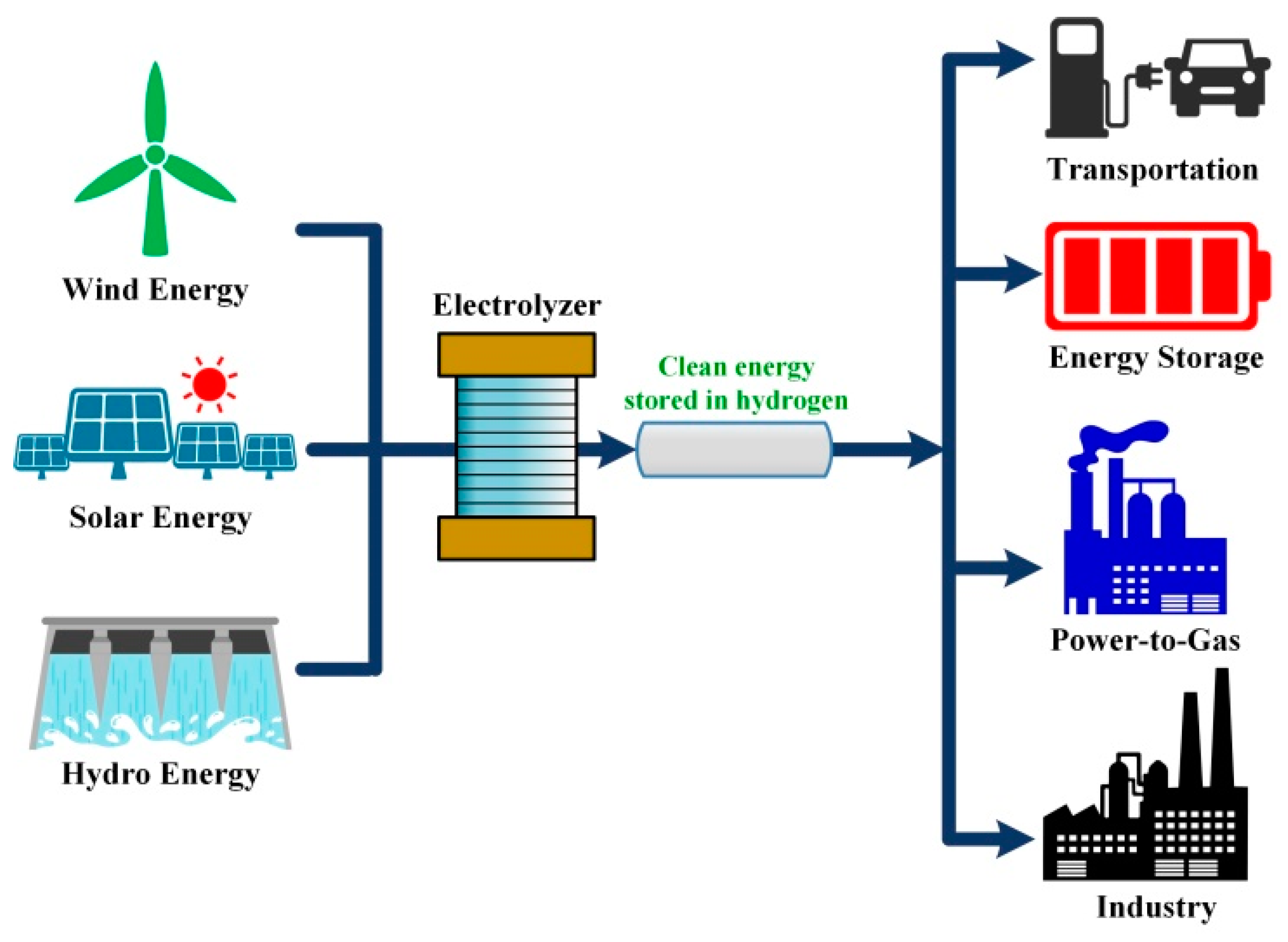
In the quest for sustainable energy solutions, Proton Exchange Membrane (PEM) electrolyzers have emerged as a pivotal technology. These systems play a critical role in the production of green hydrogen, a clean and renewable energy source. This guide provides a detailed insight into the workings of a PEM electrolyzer, explaining its components, process, and significance in the future of energy.
Understanding the Basics: What is a PEM Electrolyzer?
A PEM electrolyzer is a device that uses electricity to split water (H2O) into hydrogen (H2) and oxygen (O2) gases. This process, known as electrolysis, is facilitated by a Proton Exchange Membrane, a key component that distinguishes PEM electrolyzers from other types of electrolyzers.
The Core Components of a PEM Electrolyzer
Proton Exchange Membrane
The heart of the pem hydrogen generator work is the Proton Exchange Membrane, a specially designed membrane that only allows positively charged ions (protons) to pass through while blocking electrons. This membrane is both an electrical insulator and a conductor for protons.
Electrodes: Anode and Cathode
On either side of the membrane are two electrodes: the anode (positive electrode) and the cathode (negative electrode). These electrodes are typically made of porous materials coated with catalysts to enhance the electrolysis reaction.
Bipolar Plates
Bipolar plates are used to distribute water and electricity across the surface of the membrane. They also help in collecting the generated hydrogen and oxygen gases.
The Electrolysis Process in a PEM Electrolyzer
Water Splitting at the Anode
When electricity is applied, water molecules at the anode are split into oxygen, protons (H+ ions), and electrons. The oxygen is released as a gas, while the protons move through the membrane to the cathode.
Hydrogen Production at the Cathode
At the cathode, the protons arriving through the membrane combine with electrons (from the external circuit) to form hydrogen gas. This hydrogen gas is then collected and stored for use.
Advantages of PEM Electrolyzers
- High Purity of Hydrogen: PEM electrolyzers produce high-purity hydrogen, which is essential for applications like fuel cells.
- Compact and Modular Design: Their design allows for scalability and flexibility in installation.
- Fast Response and Dynamic Operation: PEM electrolyzers can quickly adjust to changes in electricity supply, making them ideal for pairing with intermittent renewable energy sources like wind and solar.
Challenges and Future Developments
While PEM electrolyzers are at the forefront of green hydrogen production, they face challenges such as high costs and durability concerns. The cost is primarily driven by the expensive catalyst materials (like platinum) and the membrane. Future developments are focused on finding more cost-effective materials and improving the longevity of the system.
Conclusion
PEM electrolyzers represent a key technology in the transition to a sustainable energy future. By efficiently producing green hydrogen, they offer a solution to store and utilize renewable energy, potentially transforming our energy infrastructure. As research and development continue, PEM electrolyzers are poised to play a vital role in powering a cleaner, greener world.
Top of Form